3 Bromo 2 3 Dimethylpentane
7.1: Alkyl Halides - Construction and Physical Properties
- Page ID
- 45167
Learning Objective
- allocate alkyl halides
- predict relative boiling points and solubility of alkyl halides
Introduction
Alkyl halides are also known every bit haloalkanes. Alkyl halides are compounds in which i or more hydrogen atoms in an alkane have been replaced by halogen atoms (fluorine, chlorine, bromine or iodine). We volition only look at compounds containing i halogen atom like th compounds beneath.
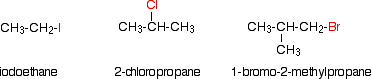
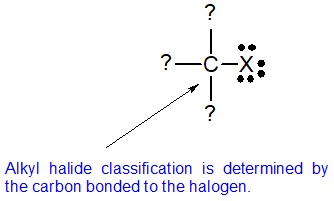
Alkyl halides fall into different classes depending on how the element of group vii cantlet is positioned on the chain of carbon atoms. Alkyl halides tin exist classified as principal, secondary, or third. The chemical reactivity of alkyl halides is frequently discussed using alkyl halide classifications to assistance discern patterns and trends. Because the neutral bonding pattern for halogens is 1 bond and three lonely pairs, the carbon and halogen ever share a single bail. Alkyl halide classification is determined past the bonding pattern of the carbon atom bonded to the element of group vii every bit shown in the diagram below.
Chief alkyl halides
In a master (ane°) haloalkane, the carbon bonded to the halogen atom is simply fastened to one other alkyl group. Some examples of primary alkyl halides include thecompounds beneath.
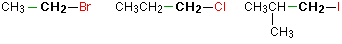
Notice that it doesn't matter how complicated the attached alkyl group is. In each instance there is only i linkage to an alkyl grouping from the CH2 group property the halogen. There is an exception to this: CH3Br and the other methyl halides are ofttimes counted equally primary alkyl halides even though at that place are no alkyl groups fastened to the carbon with the halogen on it.
Secondary alkyl halides
In a secondary (2°) haloalkane, the carbon bonded with the halogen atom is joined directly to ii other alkyl groups that can be the same or different. Some examples of secondary alkyl halides include thecompounds below.
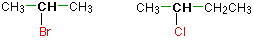
Tertiary alkyl halides
In a 3rd (3°) halogenoalkane, the carbon cantlet holding the halogen is fastened directly to three alkyl groups, which may exist any combination of same or unlike. Some examples of tertiary alkyl halides include thecompounds below.
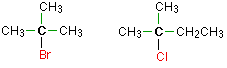
Common Names
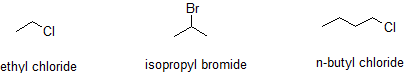
Many organic compounds are closely related to the alkanes and this similarity is incorporated into many common names. The reactions of alkanes with halogens produce halogenated hydrocarbons, compounds in which ane or more hydrogen atoms of a hydrocarbon have been replaced by element of group vii atoms: The replacement of simply one hydrogen atom gives an alkyl halide (or haloalkane). The common names of alkyl halides consist of two parts: the name of the alkyl group plus the stem of the name of the halogen, with the ending -ide.
Examples
Give the common and IUPAC names for each compound.
- CHiiiCH2CH2Br
- (CH3)2CHCl
- Give the IUPAC name for each chemical compound.
a)
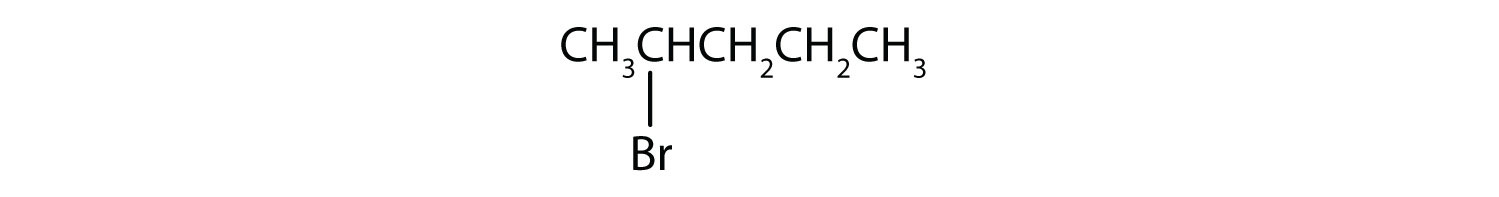
b)
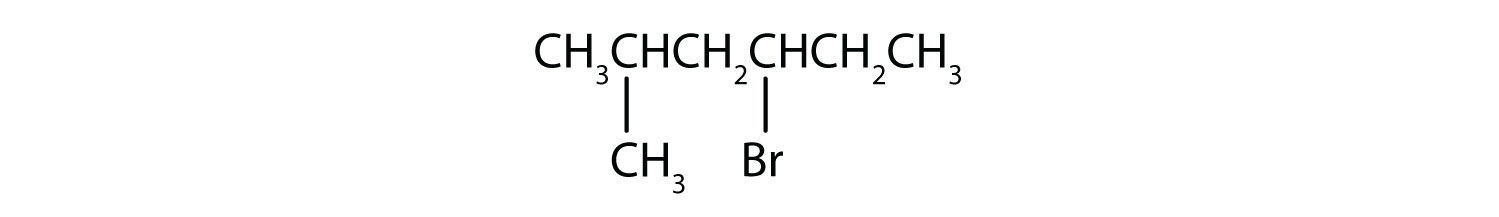
SolutionS
- The alkyl grouping (CH3CH2CH2–) is a propyl group, and the halogen is bromine (Br). The mutual name is therefore propyl bromide. For the IUPAC name, the prefix for bromine (bromo) is combined with the proper noun for a three-carbon chain (propane), preceded past a number identifying the carbon atom to which the Br atom is attached, so the IUPAC proper name is 1-bromopropane.
- The alkyl group [(CH3)2CH–] has three carbon atoms, with a chlorine (Cl) atom attached to the heart carbon atom. The alkyl group is therefore isopropyl, and the common name of the compound is isopropyl chloride. For the IUPAC proper noun, the Cl atom (prefix chloro-) fastened to the eye (second) carbon cantlet of a propane concatenation results in 2-chloropropane.
- a) The parent methane series has 5 carbon atoms in the longest continuous chain; information technology is pentane. A bromo (Br) group is attached to the second carbon atom of the concatenation. The IUPAC proper name is two-bromopentane.
b) The parent alkane series is hexane. Methyl (CH 3 ) and bromo (Br) groups are attached to the second and fourth carbon atoms, respectively. List the substituents in alphabetical order gives the name 4-bromo-2-methylhexane.
Practice
i. Give common and IUPAC names for each chemical compound.
- CH3CH2I
- CH3CHtwoCHtwoCHtwoF
2. Give the IUPAC proper name for each compound.
a)
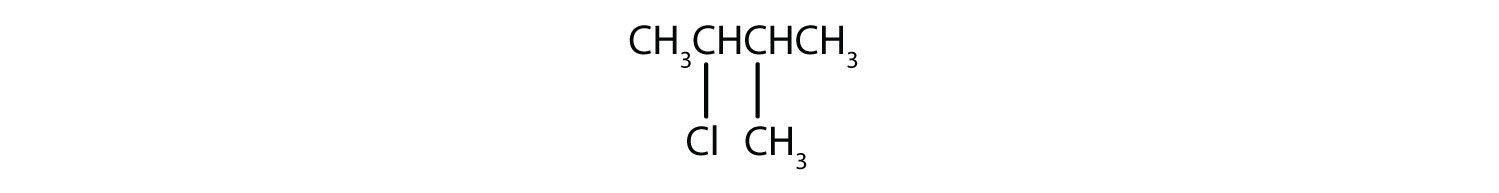
b)
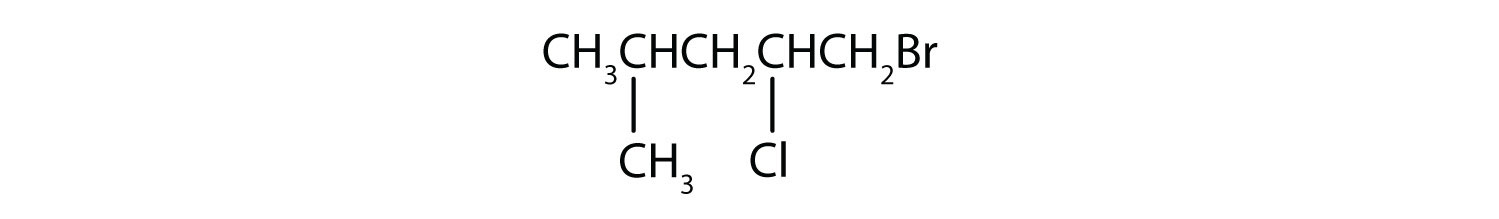
- Reply
-
i. a) ethyl iodide and iodoethane, respectively; Note the IUPAC proper noun does not need a locator number because there is only i possible structure with two carbons and ane iodine.
b) butyl fluoride and one-fluorobutane
2. a) 2-chloro-2-methylbutane
b) 1-bromo-2-chloro-4-methylpentane
Halogens and the Grapheme of the Carbon-Element of group vii Bond
With respect to electronegativity, halogens are more electronegative than carbons. This results in a carbon-halogen bond that is polarized. As shown in the image beneath, carbon cantlet has a partial positive charge, while the element of group vii has a partial negative charge.
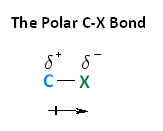
The following image shows the relationship between the halogens and electronegativity. Notice, every bit nosotros move up the periodic tabular array from iodine to fluorine, electronegativity increases.
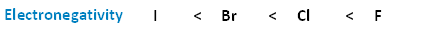
The following image shows the relationships between bail length, bond strength, and molecular size. Equally we progress downwardly the periodic table from fluorine to iodine, molecular size increases. Every bit a result, nosotros also see an increase in bond length. Conversely, equally molecular size increases and nosotros get longer bonds, the strength of those bonds decreases.

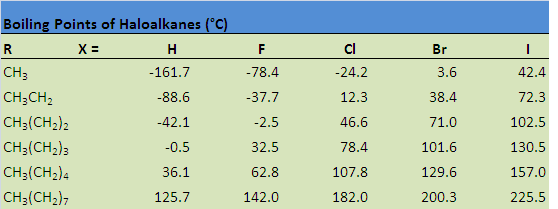
Haloalkanes Have College Boiling Points than Alkanes
When comparison alkanes and haloalkanes, we volition come across that haloalkanes have college boiling points than alkanes containing the same number of carbons. London dispersion forces are the first of two types of forces that contribute to this physical holding. You might recall from general chemical science that London dispersion forces increment with molecular expanse. In comparing haloalkanes with alkanes, haloalkanes exhibit an increase in surface surface area due to the substitution of a halogen for hydrogen. The incease in area leads to an increment in London dispersion forces, which so results in a college boiling betoken.
Dipole-dipole interaction is the second type of force that contributes to a higher boiling point. As yous may retrieve, this type of interaction is a coulombic attraction betwixt the fractional positive and partial negative charges that be between carbon-halogen bonds on carve up haloalkane molecules. Similar to London dispersion forces, dipole-dipole interactions establish a higher boiling point for haloalkanes in comparison to alkanes with the same number of carbons.

The table below illustrates how boiling points are affected by some of these properties. Notice that the boiling point increases when hydrogen is replaced past a halogen, a upshot of the increase in molecular size, as well as an increase in both London dispersion forces and dipole-dipole attractions. The boiling point also increases every bit a result of increasing the size of the halogen, equally well equally increasing the size of the carbon chain.
Solubility
Solubility in water
Alkyl halides have little to no solubility in water in spite of the polar carbon-halogen bond. The attraction between the alkyl halide molecules is stronger than the allure between the alkyl halide and water. Alkyl halides have niggling to no solubility in water, but be aware of densities. Polyhalogenated alkanes such equally dichloromethane can have densities greater than water.
Solubility in organic solvents
Alkyl halides are soluble in most organic solvents. The London Dispersion forces play a dominant role in solubility.
Exercises
Exercise
3. Classify (primary, secondary, third, vicinal, or geminal) and give the IUPAC name for the following organohalides:

4. Allocate (primary, secondary, 3rd, vicinal, or geminal) and depict the bond-line structures of the post-obit compounds:
a) two-Chloro-3,3-dimethylpentane
b) ane,1-Dichloro-four-isopropylcyclohexane
c) 3-bromo-3-ethylhexane
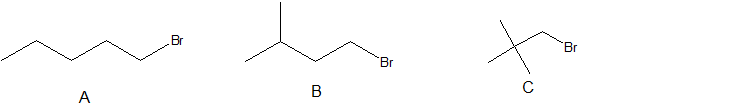
5. Arrange the following alkyl halides in order of decreasing boiling point.
-
-
vi. Predict the solvent with keen alkyl halide solubility.
-
a) water or hexane
b) water or i-octanol
c) water or benzene
d) h2o or acetone
- Solutions
-
iii.
a) secondary; v-ethyl-4-iodo-3methyl-octane
b) primary; one-bromo-2,three,4-trimethyl-pentane
c) vicinal dihalide; 4-bromo-5-chloro-2-methyl-heptane
4. (A) secondary; (B) geminal dichloride (C) tertiary
.png?revision=1&size=bestfit&width=450&height=393)
5. A > B > C
6.
a) hexane
b) benzene
c) 1-octanol
d) acetone
Alkyl halides have little to no solubility in water, but be aware of densities. Polyhalogenated alkanes tin have densities greater than h2o.
3 Bromo 2 3 Dimethylpentane,
Source: https://chem.libretexts.org/Bookshelves/Organic_Chemistry/Map%3A_Organic_Chemistry_(Wade)/07%3A_Alkyl_Halides-_Nucleophilic_Substitution_and_Elimination/7.01%3A_Alkyl_Halides_-_Structure_and_Physical_Properties
Posted by: jonesgrart1946.blogspot.com


0 Response to "3 Bromo 2 3 Dimethylpentane"
Post a Comment